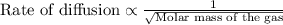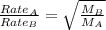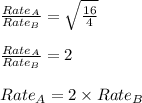Chemistry, 13.11.2019 13:31 zaniyastubbs9
Asample of gas a has a molar mass of 4 grams while a sample of gas b has a molar mass of 16 grams. which statement holds true?
both gas a and gas b diffuse at the same speed.
gas a effuses faster than gas b.
gas b effuses faster than gas a.
the molar masses of gas a and gas b are not related to effusion.
the molar mass is directly proportional to the rate of effusion.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2


Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 23.06.2019 00:00, tonimgreen17p6vqjq
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
You know the right answer?
Asample of gas a has a molar mass of 4 grams while a sample of gas b has a molar mass of 16 grams. w...
Questions in other subjects:


Mathematics, 25.11.2021 14:00


Biology, 25.11.2021 14:00


Mathematics, 25.11.2021 14:00

Chemistry, 25.11.2021 14:00