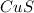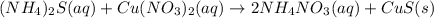Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 23.06.2019 08:00, LuvIsRage2
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2

Chemistry, 23.06.2019 14:50, aleilyg2005
Use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 +473 kj +206 kj -392 kj -91 kj -486 kj
Answers: 1

Chemistry, 23.06.2019 16:30, lfox21
What does the law of increasing costs say? a. as you make more and more of one good or service, you will also make more and more of another. b. as you make less and less of one good or service, you will also make less and less another. c. as you make more and more of one good or service, you will make less and less of another. d. there is no change in production levels when you shift from one good or service to another
Answers: 1
You know the right answer?
If aqueous solutions of ammonium sulfide and copper(ii) nitrate are mixed, which insoluble precipita...
Questions in other subjects:


Mathematics, 20.12.2020 08:20

Mathematics, 20.12.2020 08:20


English, 20.12.2020 08:20


English, 20.12.2020 08:20

Mathematics, 20.12.2020 08:20


Mathematics, 20.12.2020 08:30