

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 23.06.2019 03:00, BeeShyanne
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 05:00, daytonalive6511
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
For the reaction, calculate how many moles of the product form when 0.048 mol of o2 completely react...
Questions in other subjects:



Geography, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01




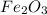 form are 0.032 moles.
form are 0.032 moles.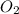 = 0.048 mole
= 0.048 mole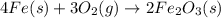
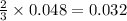 moles of
moles of 


