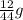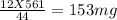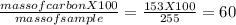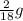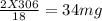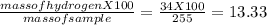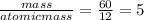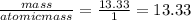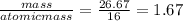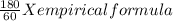Chemistry, 26.01.2020 16:31 jaymee2904p88tgh
You perform a combustion analysis on a 255 mg sample of a substance that contains only c, h, and o, and you find that 561 mg of co2 is produced, along with 306 mm of h2o.
if the substance contains only c, h, and o, what is the empirical formula
if the molar mass of the compound is 180 g/mol what is the molecular formula of the compound

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, bossboybaker
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 01:30, kaliloabousjbf
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 08:00, gomezyonathan93
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
You perform a combustion analysis on a 255 mg sample of a substance that contains only c, h, and o,...
Questions in other subjects:

English, 22.11.2020 02:50






Biology, 22.11.2020 02:50