
Chemistry, 30.09.2019 08:30 penelopymorales24
Which of the following is the change in energy of an atom emitting a photon of wavelength of 4.35 meters? [planck's constant, h = 6.626 × 10-34 js, c = 2.998 × 108 meters/second] a)1.986 × 10-25 joules b) 2.823 × 10-34 joules c)9.614 × 10-42 joules d)4.567 × 10-26 joules

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

You know the right answer?
Which of the following is the change in energy of an atom emitting a photon of wavelength of 4.35 me...
Questions in other subjects:

Mathematics, 28.06.2019 06:00




Chemistry, 28.06.2019 06:00

History, 28.06.2019 06:00

Mathematics, 28.06.2019 06:00



 joules
joules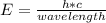 h = 6.626 × 10-34 , c = 2.998 × 108 meters/second , wavelength = 4.35
h = 6.626 × 10-34 , c = 2.998 × 108 meters/second , wavelength = 4.35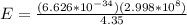 =
= 

