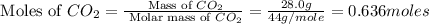Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
Calculate the amount of heat required to completely sublime 28.0 g of solid dry ice (co2 at its subl...
Questions in other subjects:


History, 20.10.2019 22:00


English, 20.10.2019 22:00



Biology, 20.10.2019 22:00



English, 20.10.2019 22:00

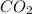 is 20.5 kJ.
is 20.5 kJ.