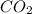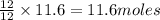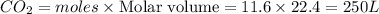In the following reaction, how many liters of carbon dioxide will be produced if
250 liters of...

Chemistry, 27.08.2019 18:00 cassiuspricerules
In the following reaction, how many liters of carbon dioxide will be produced if
250 liters of oxygen is used in the combustion of sucrose, given that both gases are at stp?
c12h22o11 + 12o2 → 12co2 + 11h2o
a. 125 liters b. 500 liters c. 250 liters d. 268.8 liters

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, leo4687
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2


Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1
You know the right answer?
Questions in other subjects:




Mathematics, 02.03.2021 23:00

History, 02.03.2021 23:00


Medicine, 02.03.2021 23:00

Mathematics, 02.03.2021 23:00

Mathematics, 02.03.2021 23:00

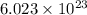 of particles.
of particles.
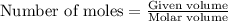
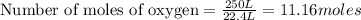
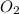 gives= 12 moles of
gives= 12 moles of