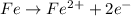Chemistry, 23.01.2020 16:31 Erinkim2292
In a chemical reaction, an iron atom became the ion fe2+. what happened to the iron atom?
a. it lost electrons and was oxidized.
b. it lost electrons and was reduced.
c. it gained electrons and was oxidized.
d. it gained electrons and was reduced.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

You know the right answer?
In a chemical reaction, an iron atom became the ion fe2+. what happened to the iron atom?
a....
a....
Questions in other subjects:


Mathematics, 22.10.2020 21:01


Mathematics, 22.10.2020 21:01


Mathematics, 22.10.2020 21:01

Biology, 22.10.2020 21:01



Mathematics, 22.10.2020 21:01

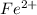 which means that iron atom by loosing two electrons from its valence shell has oxidized into positively charged ion.
which means that iron atom by loosing two electrons from its valence shell has oxidized into positively charged ion.