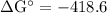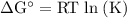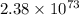Chemistry, 06.01.2020 23:31 daniella0123
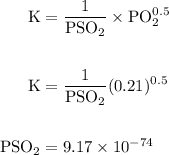
Calcium oxide is used to remove pollutant so2 from smokestack gases. the δg° of the overall reaction is -418.6 kj. what is pso2 in equilibrium with air (po2 = 0.21 atm) and solid cao?
cao(s)+so2 (g)+1⁄2o2 (g)⇔caso4 (s)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

You know the right answer?
Calcium oxide is used to remove pollutant so2 from smokestack gases. the δg° of the overall reaction...
Questions in other subjects:






Mathematics, 26.03.2021 20:20

Social Studies, 26.03.2021 20:20


Mathematics, 26.03.2021 20:20

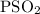 for the given reaction is
for the given reaction is 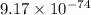 atm.
atm.