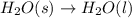The images show two processes. image a: a roasting marshmallow, image b: melting ice. which statement about the two images is true?
1. image a shows a physical change, and image b shows a chemical reaction.
2. image a shows a chemical reaction, and image b shows a physical change.
3. both images show chemical reactions that are endothermic.
4. both images show chemical reactions that are exothermic.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, saby30
Silica, sio2, is formed on silicon as an electrically insulating layer for microelectronic devices. silica is formed when silicon is exposed to o2 gas at an elevated temperature. at 900˚c, it takes 90 minutes for the oxygen to diffuse from the surface to form a 0.06 micron (0.06 x 10-6 m) thick layer of sio2 on
Answers: 1

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1


Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
The images show two processes. image a: a roasting marshmallow, image b: melting ice. which statem...
Questions in other subjects:

Mathematics, 06.01.2021 19:00

Mathematics, 06.01.2021 19:00


Mathematics, 06.01.2021 19:00


Mathematics, 06.01.2021 19:00


Mathematics, 06.01.2021 19:00


Computers and Technology, 06.01.2021 19:00

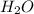 from solid to liquid form and thus is a physical change.
from solid to liquid form and thus is a physical change.