
The decomposition of calcium carbonate, caco3(s) --> cao(s) + co2(g), has the following values for free energy and enthalpy at 25.0°c.
g = 130.5 kj/mol
h = 178.3 kj/mol
what is the entropy of the reaction? use g = h – ts.
a. -160.3 j/(mol. k)
b. -47.8 j/(mol. k)
c. 160.3 j/(mol. k)
d. 1,912 j/(mol. k)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2


Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
The decomposition of calcium carbonate, caco3(s) --> cao(s) + co2(g), has the following values f...
Questions in other subjects:


English, 30.06.2019 16:00

Social Studies, 30.06.2019 16:00

Mathematics, 30.06.2019 16:00

English, 30.06.2019 16:00

History, 30.06.2019 16:00


History, 30.06.2019 16:00


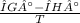
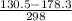 = 0.1603kJ/(mol.K) = 160.3kJ/(mol.K)
= 0.1603kJ/(mol.K) = 160.3kJ/(mol.K) 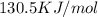
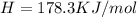
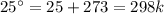
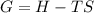
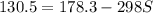

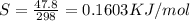
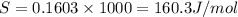 (1KJ=1000J)
(1KJ=1000J)

