
Chemistry, 25.09.2019 23:30 adrianaa34
How many moles of potassium oxide (k2o) will be formed when 1.52 moles of potassium reacts with oxygen according to the following reaction : 4 k + o2 = k2o

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Arealbot
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2
You know the right answer?
How many moles of potassium oxide (k2o) will be formed when 1.52 moles of potassium reacts with oxyg...
Questions in other subjects:


Mathematics, 25.02.2021 18:20



Mathematics, 25.02.2021 18:20

Mathematics, 25.02.2021 18:20




Mathematics, 25.02.2021 18:20

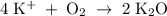
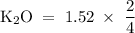 moles
moles 0.5 moles
0.5 moles

