
An equal number of moles of i2(g) and br2(g) are placed into a closed container and allowed to establish the following equilibrium:
i2(g) + br2(g) 2ibr(g)
keq = 280
which one of the following relates [ibr] to [i2] at equilibrium?
[i2] = [ibr]
[i2] < [ibr]
[i2] = 2 [ibr]
[i2] = 280 [ibr]

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2
You know the right answer?
An equal number of moles of i2(g) and br2(g) are placed into a closed container and allowed to estab...
Questions in other subjects:




Mathematics, 17.11.2020 18:30



Engineering, 17.11.2020 18:30



![[I_2]](/tpl/images/0244/0546/a6faa.png)
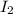 = Moles of
= Moles of 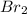
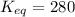
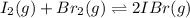
![K_{eq}=\frac{[IBr]^2}{[I_2][Br_2]}](/tpl/images/0244/0546/86934.png)
![[IBr]](/tpl/images/0244/0546/f07e4.png) and
and ![[I_2]](/tpl/images/0244/0546/a1c01.png) .
.![280=\frac{[IBr]^2}{[I_2][I_2]}](/tpl/images/0244/0546/a9dc3.png)
![280=\frac{[IBr]^2}{[I_2]^2}](/tpl/images/0244/0546/b309d.png)
![\sqrt{280}=\frac{[IBr]}{[I_2]}](/tpl/images/0244/0546/a31b5.png)
![[IBr]=16.733\times [I_2]](/tpl/images/0244/0546/79021.png)
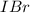 is greater than the concentration of
is greater than the concentration of 

