
Chemistry, 28.01.2020 21:58 alaina3792
When a person has excess stomach acid, he or she may ingest some sodium bicarbonate. the rationale for doing this is that the sodium bicarbonate is:
a base and will neutralize the excess acid
an acid and will weaken the excess acid
neutral and will dilute the excess acid
it will make the person thirsty; they will drink water and dilute the excess acid.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:10, hadellolo8839
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
When a person has excess stomach acid, he or she may ingest some sodium bicarbonate. the rationale f...
Questions in other subjects:

History, 27.09.2019 07:10


English, 27.09.2019 07:10


Mathematics, 27.09.2019 07:10

Mathematics, 27.09.2019 07:10

English, 27.09.2019 07:10



English, 27.09.2019 07:10

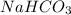 . It is basic in nature as it dissociates to give sodium and bicarbonate ions.
. It is basic in nature as it dissociates to give sodium and bicarbonate ions. 

