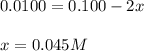10.0 ml of a 0.100 mol l–1 solution of a metal ion m2+ is mixed with 10.0 ml of a 0.100 mol l–1 solution of a substance l. the following equilibrium is established:
m2+(aq) + 2l(aq) picture ml22+(aq)
at equilibrium the concentration of l is found to be 0.0100 mol l–1. what is the equilibrium concentration of ml22+, in mol l–1?
someone me

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1


Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
10.0 ml of a 0.100 mol l–1 solution of a metal ion m2+ is mixed with 10.0 ml of a 0.100 mol l–1 solu...
Questions in other subjects:


Mathematics, 05.03.2020 02:22








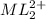 at equilibrium is 0.045 M.
at equilibrium is 0.045 M.![[M^{2+}]_{initial}=0.100M](/tpl/images/0204/7051/8debc.png)
![[L]_{initial}=0.100M](/tpl/images/0204/7051/9e7cd.png)

![[L]_{eqllm}=0.0100M](/tpl/images/0204/7051/18682.png)