
Chemistry, 09.01.2020 08:31 keigleyhannah30
In the first law of thermodynamics, △e = q-w, what does △e stand for?
a. the work done by the piston
b. the internal energy of the system
c. the heat added to the system
d. the change in the internal energy of the system

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
In the first law of thermodynamics, △e = q-w, what does △e stand for?
a. the work done...
a. the work done...
Questions in other subjects:

Biology, 06.03.2021 09:10

Mathematics, 06.03.2021 09:10

Computers and Technology, 06.03.2021 09:10

History, 06.03.2021 09:10

English, 06.03.2021 09:10



English, 06.03.2021 09:10


Social Studies, 06.03.2021 09:10

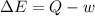
 represents the internal energy of the system.
represents the internal energy of the system.

