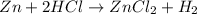When zinc is placed in hydrochloric acid, a gas is produced. when the gas is collected in a tube and a lit match is placed in the tube, a pop is heard. this is a test for hydrogen gas. what can be concluded from this observations. a) a change in state has occurred. b) the zinc metal was turned into hydrogen gas. c) a chemical change has occurred producing the hydrogen gas. d) a physical change has occurred producing the hydrogen gas.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, megaaan214p61pb7
Which compounds have the empirical formula ch2o? a. c2h4o2 b. c3h6o3 c. ch2o2 d. c5h10o5 e. c6h12o6
Answers: 3


Chemistry, 22.06.2019 11:00, familyvazquez7
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
When zinc is placed in hydrochloric acid, a gas is produced. when the gas is collected in a tube and...
Questions in other subjects:

Biology, 07.12.2019 09:31


Mathematics, 07.12.2019 09:31

Mathematics, 07.12.2019 09:31

Biology, 07.12.2019 09:31

Mathematics, 07.12.2019 09:31


English, 07.12.2019 09:31


Mathematics, 07.12.2019 09:31