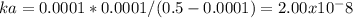12. which compound can act as both a brønstedlowry
acid and a brønstedlowry
base? (1 po...

Chemistry, 04.10.2019 23:00 montecillolinda
12. which compound can act as both a brønstedlowry
acid and a brønstedlowry
base? (1 point)
water
ammonia
sodium hydroxide
hyrdrochloric acid
13. what are the acids in the following equilibrium reaction?
cn– + h2o hcn + oh–
(1 point)
cn–, h2o
h2o, hcn
cn–, oh–
h2o, oh–
14. the products of selfionization
of water are (1 point)
h3o+ and h2o
oh– and oh+
oh+ and h–
oh– and h+
15. which type of solution is one with a ph of 8? (1 point)
acidic
basic
neutral
the type varies, depending on the solution.
16. the acid dissociation constant for an acid dissolved in water is equal to the (1 point)
equilibrium constant
equilibrium constant times the concentration of water
equilibrium constant divided by the concentration of water
equilibrium constant times the equilibrium constant of water
17. a 0.12 m solution of an acid that ionizes only slightly in solution would be termed (1 point)
concentrated and weak
strong and dilute
dilute and weak
concentrated and strong
essay
18. if the solubility of a gas is 7.5 g/l at 404 kpa pressure, what is the solubility of the gas when
the pressure is 202 kpa? show your work.
(3 points)
19. explain on a particle basis how the addition of a solute affects the boiling point, the freezing
point, and the vapor pressure of the solvent.
(6 points)
20.
calculate the hydrogenion
concentration [h+] for the aqueous solution in which [oh–] is 1 x
10–11 mol/l. is this solution acididc, basic, or neutral? show your work.
(3 points)
21. calculate the acid dissociation constant of a weak monoprotic acid if a 0.5m solution of this
acid gives a hydrogenion
concentration of 0.000 1m? show your work.
hint: monoprotic means containing one proton.
(3 points)
22. compare and contrast the properties of acids and bases. include two similarities and two
differences.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, gallegosarmanni
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1


Chemistry, 22.06.2019 14:30, jessiereyes2924
What is the relationship between wind and ocean waves? question 17 options: wind moving at higher speeds will transfer more energy to the water, resulting in stronger waves. wind moving at higher speeds will transfer energy over a larger part of the ocean water, resulting in waves with a shorter wavelength. winds moving at higher speeds with cause water to move forward at faster rates, causing larger ocean waves. winds moving at higher speeds will affect deeper water, resulting in waves that move at a faster rate. how do temperature and salinity affect deepwater currents? question 15 options: as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
Questions in other subjects:


Mathematics, 22.06.2021 18:50


Mathematics, 22.06.2021 18:50




Mathematics, 22.06.2021 18:50

Mathematics, 22.06.2021 18:50

![[H+][OH-]= Kw = 1.0 * 10^-14](/tpl/images/0287/2817/b9e7e.png)
![[H+]= Kw/ [OH-]= 1.0x 10^-14 / 1 x 10^-11 =1 x 10^-3 mol/L pH = - log [H+]= - log 1 x 10^-3 = 3](/tpl/images/0287/2817/63f2b.png)
![ka = [H]^2 / (0.5 - [H+]) ](/tpl/images/0287/2817/777b7.png)