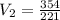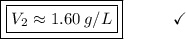Chemistry, 30.10.2019 12:31 jessicamcummins
The gas in a cylinder has a volume of 3 liters at a pressure of 118 kpa. the pressure of the gas is increased to 221 kpa. assuming the temperature remains constant, what would the new volume be?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2


Chemistry, 23.06.2019 06:00, kristine2424
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
You know the right answer?
The gas in a cylinder has a volume of 3 liters at a pressure of 118 kpa. the pressure of the gas is...
Questions in other subjects:

Chemistry, 16.04.2020 11:44

Physics, 16.04.2020 11:44



Arts, 16.04.2020 11:44



Mathematics, 16.04.2020 11:56

Mathematics, 16.04.2020 11:56

Biology, 16.04.2020 11:56