Chemistry, 15.01.2020 12:31 raewalker23p4ibhy
What is the definition of a common ion as it applies to le chatelier’s principle?
a) an ion that is found in a wide variety of compounds
b) an ion that is present in every sample of pure water
c) an ion that is found dissolved in most aqueous solutions that are widely used in labs
d) an ion that is present in an equilibrium system and a compound added to the system

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1

Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 22:30, itsmaddierae11
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
What is the definition of a common ion as it applies to le chatelier’s principle?
a) an ion t...
a) an ion t...
Questions in other subjects:



Mathematics, 03.02.2020 10:59


Mathematics, 03.02.2020 10:59

Mathematics, 03.02.2020 10:59



Mathematics, 03.02.2020 10:59

Advanced Placement (AP), 03.02.2020 10:59

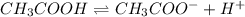
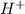 as common ion.
as common ion.