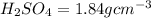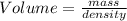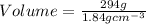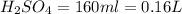2. sulfur dioxide gas (so2) reacts with excess oxygen gas (o2) and excess liquid water (h2o) to form liquid sulfuric acid (h2so4) in the unbalanced equation below: so2 + o2 + h2o h2so4 in the laboratory, a chemist carries out this reaction at stp with 67.2 l of sulfur dioxide (so2). how many liters of h2so4 did the chemist produce? 1 mole of any gas = 22.4 l of that same gas at stp • part a: write a balanced equation for the reaction. • part b: calculate the number of liters of h2so4 produced.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mayamabjishovrvq9
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 06:00, Kjswagout5052
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
2. sulfur dioxide gas (so2) reacts with excess oxygen gas (o2) and excess liquid water (h2o) to form...
Questions in other subjects:

Mathematics, 16.04.2020 22:57

History, 16.04.2020 22:57








Spanish, 16.04.2020 22:57

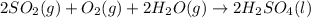
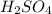 =0.16L
=0.16L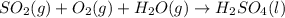
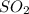 occupies 22.4 L at STP
occupies 22.4 L at STP 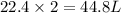 and produce 2 moles of
and produce 2 moles of 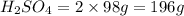
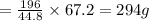 of
of