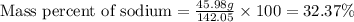Chemistry, 17.11.2019 04:31 angelalovelis
What is the percent by weight of sodium in sodium sulfate (na2so4)? (the molar mass of na = 22.99, s = 32.07, and o = 16.00.) 16.18% 22.58% 32.37% 45.05% 51.17%

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3


Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 01:50, UncleVictor5188
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
What is the percent by weight of sodium in sodium sulfate (na2so4)? (the molar mass of na = 22.99,...
Questions in other subjects:

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Spanish, 18.09.2020 14:01

Physics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

English, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

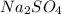 , there are 2 sodium atoms, 1 sulfur atom and 4 oxygen atoms.
, there are 2 sodium atoms, 1 sulfur atom and 4 oxygen atoms.
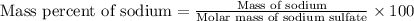
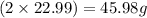
![[(2\times 22.99)+32.07+(4\times 16)]=142.05g](/tpl/images/0378/0552/0740c.png)