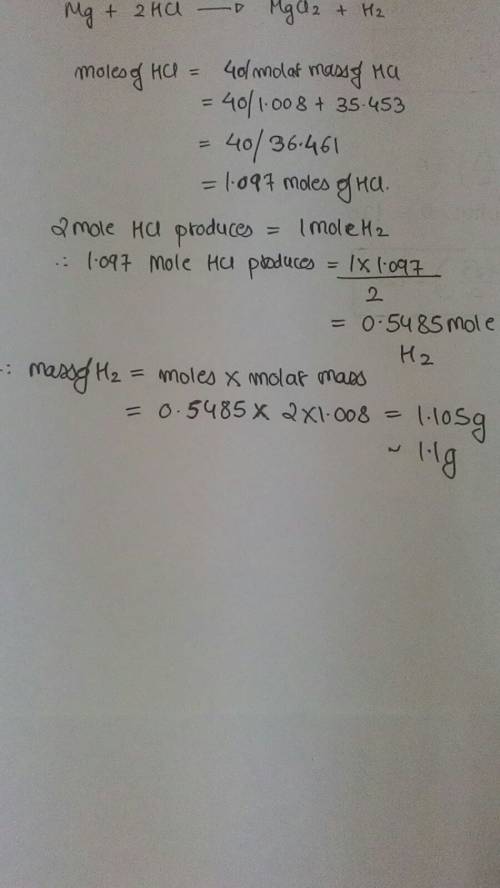Chemistry, 16.11.2019 04:31 idontknow113
If 40.0 g of hcl react with an excess of magnesium metal, what is the theoretical yield of hydrogen?
1.11 g
2.22 g
52.2 g
104 g

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
If 40.0 g of hcl react with an excess of magnesium metal, what is the theoretical yield of hydrogen?...
Questions in other subjects:



Physics, 19.03.2020 21:06




English, 19.03.2020 21:06

Chemistry, 19.03.2020 21:06

History, 19.03.2020 21:06