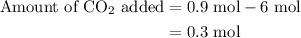Chemistry, 30.01.2020 05:57 Queiao4088
An equilibrium mixture contains 0.600 mol of each of the products (carbon dioxide and hydrogen gas) and 0.200 mol of each of the reactants (carbon monoxide and water vapor) in a 1.00-l container. how many moles of carbon dioxide would have to be added at constant temperature and volume to increase the amount of carbon monoxide to 0.300 mol once equilibrium has been reestablished?

Answers: 2


Other questions on the subject: Chemistry



You know the right answer?
An equilibrium mixture contains 0.600 mol of each of the products (carbon dioxide and hydrogen gas)...
Questions in other subjects:



History, 05.03.2021 19:00

English, 05.03.2021 19:00



Mathematics, 05.03.2021 19:00



Mathematics, 05.03.2021 19:00

 of
of  are added so as to increase the amount of carbon monoxide to 0.3 mol.
are added so as to increase the amount of carbon monoxide to 0.3 mol.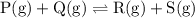
![{\text{K}}=\dfrac{{\left[ {\text{R}} \right]\left[ {\text{S}}\right]}}{{\left[{\text{P}}\right]\left[ {\text{Q}} \right]}}](/tpl/images/0484/7673/9b899.png)
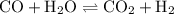
![{\text{K = }}\dfrac{{\left[ {{\text{C}}{{\text{O}}_{\text{2}}}} \right]\left[{{{\text{H}}_{\text{2}}}} \right]}}{{\left[ {{\text{CO}}}\right]\left[{{{\text{H}}_2}{\text{O}}} \right]}}](/tpl/images/0484/7673/6dcad.png) .......(1)
.......(1)![\left[{{\text{C}}{{\text{O}}_{\text{2}}}}\right]](/tpl/images/0484/7673/9014c.png) is the concentration of carbon dioxide.
is the concentration of carbon dioxide.
![\left[{{{\text{H}}_{\text{2}}}}\right]](/tpl/images/0484/7673/340fe.png) is the concentration of hydrogen.
is the concentration of hydrogen.
![\left[{{\text{CO}}}\right]](/tpl/images/0484/7673/d6da7.png) is the concentration of carbon monoxide.
is the concentration of carbon monoxide.
![\left[{{{\text{H}}_2}{\text{O}}}\right]](/tpl/images/0484/7673/62a9e.png) is the concentration of water.
is the concentration of water.
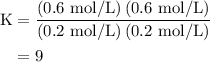
![\left[{{\text{C}}{{\text{O}}_{\text{2}}}}\right]=\dfrac{{{\text{K}}\left( {\left[{{\text{CO}}} \right]\left[{{{\text{H}}_2}{\text{O}}}\right]}\right)}}{{\left[{{{\text{H}}_{\text{2}}}} \right]}}](/tpl/images/0484/7673/f6940.png) ......(2)
......(2)![\begin{aligned}\left[ {{\text{C}}{{\text{O}}_{\text{2}}}}\right]&= \frac{{{\text{9}}\left( {{\text{0}}{\text{.3 mol/L}}}\right)\left({{\text{0}}{\text{.2 mol/L}}}\right)}}{{{\text{0}}{\text{.6 mol/L}}}}\\&= 0.{\text{9 mol/L}}\\\end{aligned}](/tpl/images/0484/7673/95ec6.png)