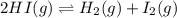Chemistry, 19.11.2019 17:31 mattydoug4818
Two experiments were performed involving the following equilibrium. the temperature was the same in both experiments. h2(g) + i2(g) 2hi(g) in experiment a, 1.0 m i2 and 1.0 m h2 were initially added to a flask and equilibrium was established. in experiment b, 2.0 m hi was initially added to a second flask and equilibrium was established. which of the following statements is always true about the equilibrium concentrations?
a.[h2] equals [hi] in experiment a.
b.[hi] equals 2[h2] in experiment a.
c.[hi] in experiment a equals [hi] in experiment b.
d.[hi] in experiment a equals 1/2[i2] in experiment b.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mvtthewisdead
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

You know the right answer?
Two experiments were performed involving the following equilibrium. the temperature was the same in...
Questions in other subjects:

Mathematics, 15.04.2021 05:50

Mathematics, 15.04.2021 05:50



History, 15.04.2021 05:50

Mathematics, 15.04.2021 05:50

Mathematics, 15.04.2021 05:50

Biology, 15.04.2021 05:50

Mathematics, 15.04.2021 05:50

Mathematics, 15.04.2021 05:50