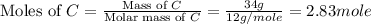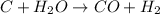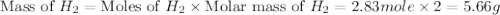Chemistry, 12.01.2020 19:31 ausemkattom3034
How many grams of h2 would be formed if 34 grams of carbon reacted with an unlimited amount of h2o? the reaction is:
c + h2o → co + h2
the atomic mass of c is 12.01 g/mole. the atomic mass of h2 is 2.016 g/mole. finish the problem by choosing the correct format for dimensional analysis.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, jude3412
In an effort to address concerns about global warming, a power plant in portland, oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2
You know the right answer?
How many grams of h2 would be formed if 34 grams of carbon reacted with an unlimited amount of h2o?...
Questions in other subjects:

Mathematics, 10.12.2020 19:50

English, 10.12.2020 19:50



Mathematics, 10.12.2020 19:50


Mathematics, 10.12.2020 19:50

Mathematics, 10.12.2020 19:50

English, 10.12.2020 19:50

Mathematics, 10.12.2020 19:50

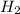 will be, 5.66 grams
will be, 5.66 grams