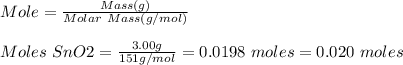Chemistry, 03.02.2020 21:02 alyssamaize
Base your answer to the question on the information below and on your knowledge of chemistry. at 1023 k and 1 atm, a 3.00-gram sample of sno2(s) (gram formula mass = 151 g/mol) reacts with hydrogen gas to produce tin and water, as shown in the balanced equation below. sno2(s) + 2h2(g) → sn(l) + 2h2o(g) show a numerical setup for calculating the number of moles of sno2(s) in the 3.00-gram sample.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 21:30, rileydavidharless
Which substance can be broken down by chemical means
Answers: 1
You know the right answer?
Base your answer to the question on the information below and on your knowledge of chemistry. at 102...
Questions in other subjects:

History, 14.07.2019 03:30

Health, 14.07.2019 03:30

Mathematics, 14.07.2019 03:30

Geography, 14.07.2019 03:30

Mathematics, 14.07.2019 03:30


Biology, 14.07.2019 03:30

Mathematics, 14.07.2019 03:30

Mathematics, 14.07.2019 03:30

Physics, 14.07.2019 03:30