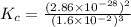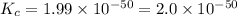Chemistry, 24.09.2019 13:30 genyjoannerubiera
At 298 k, the equilibrium concentration of o2 is 1.6 x 10-2 m, and the equilibrium concentration of o3 is 2.86 x 10-28 m. what is the equilibrium constant of the reaction at this temperature?
a. 2.0*10∧-50
b. 2.0*10∧50
c. 1.8*10∧-26
d. 1.8*10∧26


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, ameliaxbowen7
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1



Chemistry, 23.06.2019 11:30, nickolasbradyp0hvwl
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3
You know the right answer?
At 298 k, the equilibrium concentration of o2 is 1.6 x 10-2 m, and the equilibrium concentration of...
Questions in other subjects:



English, 16.09.2021 05:30

Mathematics, 16.09.2021 05:30

English, 16.09.2021 05:30

Mathematics, 16.09.2021 05:30

Mathematics, 16.09.2021 05:30

Mathematics, 16.09.2021 05:30

Mathematics, 16.09.2021 05:30

Mathematics, 16.09.2021 05:30

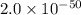
 =
= 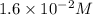
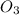 =
= 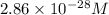
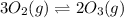
![K_c=\frac{[O_3]^2}{[O_2]^3}](/tpl/images/0258/2319/13f9f.png)