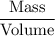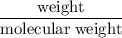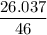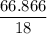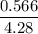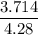Chemistry, 22.12.2019 01:31 alexmoy45p8yd7v
Asolution is made by mixing 33.0 ml of ethanol, c2h6o and 67.0 ml of water. assuming ideal behavior, what is the vapor pressure of the solution at 20 degree c? ethanol= .789 g/ml 43.9 torr. water= .998 g/ml 17.5 torr

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, shreyapatel2004
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Asolution is made by mixing 33.0 ml of ethanol, c2h6o and 67.0 ml of water. assuming ideal behavior,...
Questions in other subjects:



Mathematics, 06.03.2021 01:00

Mathematics, 06.03.2021 01:00

Social Studies, 06.03.2021 01:00

Mathematics, 06.03.2021 01:00

Chemistry, 06.03.2021 01:00

Mathematics, 06.03.2021 01:00


 Partial pressure
Partial pressure