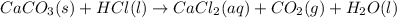Chemistry, 31.01.2020 12:49 viviansotelo12
Which answer below correctly identifies the type of change and the explanation for the mixture of calcium corbonate and hydrochloric acid?
physical change because even though gas formation was observed, the calcium carbonate retained its original properties.
physical change because even though a particle size change was observed, the calcium retained its original properties.
chemical change because gas formation was observed, which indicated that the calcium carbonate was transformed into a different substance.
chemical change because a particle size change was observed, which indicated that the calcium carbonate was transformed into a different substance.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, cutebab4786
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
Which answer below correctly identifies the type of change and the explanation for the mixture of ca...
Questions in other subjects:



Chemistry, 30.01.2021 04:30

Chemistry, 30.01.2021 04:30

Chemistry, 30.01.2021 04:30

Chemistry, 30.01.2021 04:30

Mathematics, 30.01.2021 04:30


Advanced Placement (AP), 30.01.2021 04:30