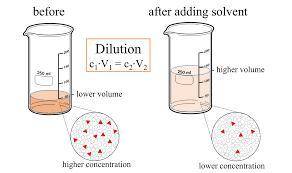
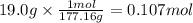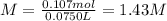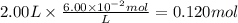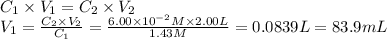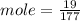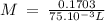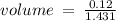On part "c": the forensic technician at a crime scene has just prepared a luminol stock solution by adding 19.0g of luminol into a total volume of 75.0ml of h2o.
a)what is the molarity of the stock solution of luminol?
anwer i got: molarity of luminol solution = 1.43m b)before investigating the scene, the technician must dilute the luminol solution to a concentration of 6.00×10−2 m. the diluted solution is then placed in a spray bottle for application on the desired surfaces.
i cannot get the correct answer for "c" have tried: 172ml,11.9ml, and 1.19*10^4. the only other possibility that i can come up with is: 83.9ml. would this one be i still completely out to
c)how many moles of luminol are present in 2.00 l of the diluted spray?
anwer i got: moles of luminol = 0.120mol what volume of the stock solution (part a) would contain the number of moles present in the diluted solution (part b)?
express your answer in milliliters.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 21.06.2019 23:00, jasmineharris121
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
You know the right answer?
On part "c": the forensic technician at a crime scene has just prepared a luminol stock solution by...
Questions in other subjects:

Mathematics, 14.12.2020 22:20

History, 14.12.2020 22:20



Biology, 14.12.2020 22:20

Mathematics, 14.12.2020 22:20




Mathematics, 14.12.2020 22:20