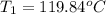Apiece of glass with a mass of 32.50 g specific heat of 0.840 j/g*°c and an initial temperature of 115 °c was dropped into a calorimeter containing 57 g of water (specific heat 4.184 j/g*°c). the final temperature of the glass and water in the calorimeter was 119.2 °c. what was the initial temperature of the water?
39.84°c
79.68°c
119.84°c
139.68°c

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, eweqwoewoji
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 18:00, kingamir
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Apiece of glass with a mass of 32.50 g specific heat of 0.840 j/g*°c and an initial temperature of 1...
Questions in other subjects:

Mathematics, 13.11.2020 17:30

Mathematics, 13.11.2020 17:30

Mathematics, 13.11.2020 17:30

Mathematics, 13.11.2020 17:30



Social Studies, 13.11.2020 17:30



Chemistry, 13.11.2020 17:30

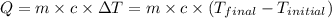
![m_1\times c_g\times (T_{final}-T_2)=-[m_2\times c_w\times (T_{final}-T_1)]](/tpl/images/0305/5772/96ec8.png) .................(1)
.................(1)
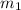 = mass of glass = 32.50 g
= mass of glass = 32.50 g
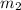 = mass of water = 57 g
= mass of water = 57 g
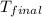 = final temperature of water and glass =
= final temperature of water and glass = 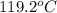
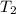 = initial temperature of water = ?
= initial temperature of water = ?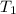 = initial temperature glass =
= initial temperature glass = 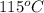
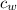 = specific heat of water =
= specific heat of water = 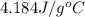
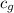 = specific heat of glass =
= specific heat of glass = 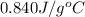
![(32.50g)\times (0.840J/g^oC)\times (119.2^oC-115^oC)=-[(57g)\times (4.184J/g^oC)\times (119.2^oC-T_1)]](/tpl/images/0305/5772/c0a00.png)