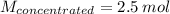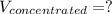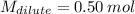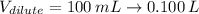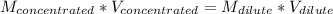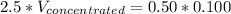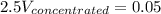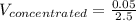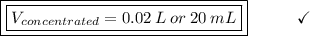Answer the question below. type your response in the space provided. what volume of a 2.5 m stock solution of acetic acid (hc2h3o2) is required to prepare 100.0 milliliters of a 0.50 m acetic acid solution? use the equation mconcentrated*vconcentrated = mdilute*vdilute.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, fordkenae
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2


Chemistry, 23.06.2019 00:30, quintink
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
Answer the question below. type your response in the space provided. what volume of a 2.5 m stock so...
Questions in other subjects:

History, 08.04.2020 21:41

English, 08.04.2020 21:41


Social Studies, 08.04.2020 21:41



Mathematics, 08.04.2020 21:41

History, 08.04.2020 21:41