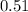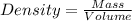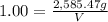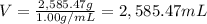Chemistry, 01.09.2019 22:30 emmilicious
For a caffeinated drink with a caffeine mass percent of 0.51% and a density of 1.00 g/ml, how many ml of the drink would be required to reach an ld50 of 170 mg/kg body mass if the person weighed 171 lb?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, Brooke7644
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2


Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

You know the right answer?
For a caffeinated drink with a caffeine mass percent of 0.51% and a density of 1.00 g/ml, how many m...
Questions in other subjects:

Mathematics, 09.03.2022 15:40

Mathematics, 09.03.2022 15:40


Mathematics, 09.03.2022 15:40

Biology, 09.03.2022 15:40


Mathematics, 09.03.2022 15:40

Mathematics, 09.03.2022 15:40

English, 09.03.2022 15:40