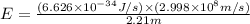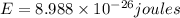Calculate the change in energy of an atom that emits a photon of wavelength 2.21 meters. (planck’s constant is 6.626 x 10-34 joule seconds, the speed of light is 2.998 x 108 m/s)
* 8.9886 x10-26 joules
*4.8844 x 10-42 joules second
* 1.9864 x 10-25 joules
* 1.4643 x 10-33 joules /second

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
Calculate the change in energy of an atom that emits a photon of wavelength 2.21 meters. (planck’s c...
Questions in other subjects:




Mathematics, 10.02.2021 07:10

Mathematics, 10.02.2021 07:10

Mathematics, 10.02.2021 07:10

Mathematics, 10.02.2021 07:10

Chemistry, 10.02.2021 07:10


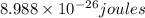
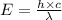
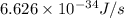
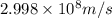
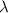 = wavelength = 2.21 m
= wavelength = 2.21 m