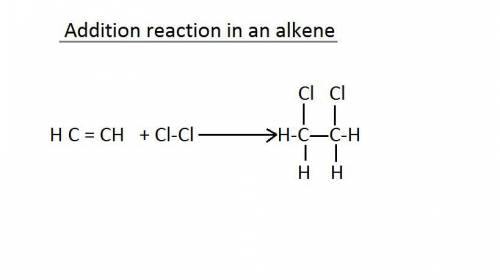
In an addition reaction, which bond of the reactant is broken?
a. carbon carbon single bond...

Chemistry, 02.10.2019 06:30 mimireds5419
In an addition reaction, which bond of the reactant is broken?
a. carbon carbon single bond
c. carbon carbon double bond
b. carbon hydrogen single bond
d. carbon hydrogen double bondin chemistry, a hydrogen bond is a type of attractive intermolecular force that exists between two partial electric charges of opposite polarity. [ although stronger than most other intermolecular forces, the hydrogen bond is much weaker than both the ionic bond and the covalent bond. ]

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 22:30, creepycrepes
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
Questions in other subjects:






Geography, 30.03.2021 03:50



Mathematics, 30.03.2021 03:50

Physics, 30.03.2021 03:50