
Chemistry, 29.01.2020 00:52 waterdrop026
The reaction ab(aq)→a(g)+b(g) is second order in ab and has a rate constant of 0.0164 m −1 ⋅ s −1 at 25.0 ∘ c . a reaction vessel initially contains 250.0 ml of 0.104 m ab which is allowed to react to form the gaseous product. the product is collected over water at 25.0 ∘ c . part a how much time is required to produce 142.0 ml of the products at a barometric pressure of 707.3 mmhg . (the vapor pressure of water at this temperature is 23.8 mmhg .)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:10, angellong94
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

You know the right answer?
The reaction ab(aq)→a(g)+b(g) is second order in ab and has a rate constant of 0.0164 m −1 ⋅ s −1 at...
Questions in other subjects:


English, 06.09.2021 19:00

Mathematics, 06.09.2021 19:00


Business, 06.09.2021 19:00

Mathematics, 06.09.2021 19:00

Geography, 06.09.2021 19:00


Social Studies, 06.09.2021 19:00

![v=k[AB]^2](/tpl/images/0479/4220/bde1b.png) . In the statement, we obtain that
. In the statement, we obtain that ![[AB]=0.104~M](/tpl/images/0479/4220/4f567.png) and, at 25 ºC,
and, at 25 ºC, 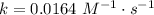 . Then:
. Then:![v=k[AB]^2\\\\ v=0.0164\cdot0.104^2\\\\ v=0.0164\cdot0.010816\\\\ v\approx0.000177=1.77\times10^{-4}~mol/s](/tpl/images/0479/4220/68b4c.png)
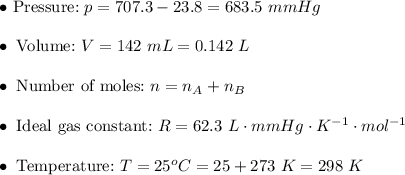

 . Hence:
. Hence: 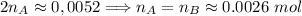 .
.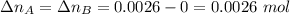 . Furthermore, since the ratio of AB to A and to B is 1:1,
. Furthermore, since the ratio of AB to A and to B is 1:1, 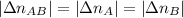 .
.![v=\dfrac{|\Delta[AB]|}{\Delta t}=\dfrac{1}{\Delta t}\cdot\dfrac{|\Delta n_{AB}|}{V}=\dfrac{1}{\Delta t}\cdot\dfrac{|\Delta n_A||}{250~mL}\\\\ 1.77\cdot10^{-4}=\dfrac{1}{\Delta t}\cdot\dfrac{0.0026~mol}{0.25~L}\\\\ \Delta t=\dfrac{0.0026}{0.25\cdot1.77\cdot10^{-4}}\\\\ \boxed{\Delta t\approx58.76~s}](/tpl/images/0479/4220/8e656.png)



