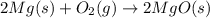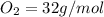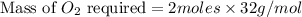Chemistry, 23.01.2020 14:31 blessed4628
The chemical equation below shows the burning of magnesium (mg) with oxygen (o2) to form magnesium oxide (mgo).
2mg + o2 mc009-1.jpg 2mgo
the molar mass of o2 is 32.0 g/mol. what mass, in grams, of o2 is required to react completely with 4.00 mol of mg?
2.00

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
The chemical equation below shows the burning of magnesium (mg) with oxygen (o2) to form magnesium o...
Questions in other subjects:

Health, 16.12.2021 01:30

English, 16.12.2021 01:30

Geography, 16.12.2021 01:30

Mathematics, 16.12.2021 01:30

Mathematics, 16.12.2021 01:30



Mathematics, 16.12.2021 01:30


 will be required to react completely with 4 moles of Mg.
will be required to react completely with 4 moles of Mg.