
Chemistry, 28.01.2020 20:49 cruzsuarezjanca
Consider the following equilibrium system: 3o2(g) 2o3(g); keq = 1 which equation compares the concentration of oxygen and ozone?
a. [o2]3 = [o3]2
b. [o2] = [o3]
c. [o2] = [o3]3/2
d. [o2]2 = [o3]3

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, carog24
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1

Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
Consider the following equilibrium system: 3o2(g) 2o3(g); keq = 1 which equation compares the conc...
Questions in other subjects:




English, 05.05.2020 03:33

Mathematics, 05.05.2020 03:33

English, 05.05.2020 03:33

Mathematics, 05.05.2020 03:33

Biology, 05.05.2020 03:33


Mathematics, 05.05.2020 03:33

![[O_2]^3=[O_3]^2](/tpl/images/0478/4387/0183b.png)
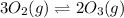
![K_{eq}=\frac{[O_3]^2}{[O_2]^3}](/tpl/images/0478/4387/1b55f.png)
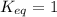
![1=\frac{[O_3]^2}{[O_2]^3}](/tpl/images/0478/4387/c7e88.png)


