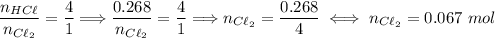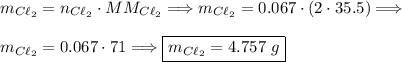4hcl + mno2 → mncl2 + 2h2o + cl2 .

Chemistry, 26.10.2019 04:43 finesser16
Consider the following chemical reaction:
4hcl + mno2 → mncl2 + 2h2o + cl2 .
when 0.268 mol of hcl reacts stoichiometrically with mno2, how many grams of cl2 is produced?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, monnicawilliam
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1


Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1
You know the right answer?
Consider the following chemical reaction:
4hcl + mno2 → mncl2 + 2h2o + cl2 .
4hcl + mno2 → mncl2 + 2h2o + cl2 .
Questions in other subjects:





Mathematics, 26.03.2020 20:29

Mathematics, 26.03.2020 20:29




 to
to  is
is 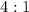 . Then:
. Then: