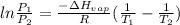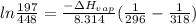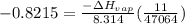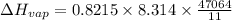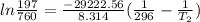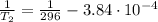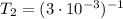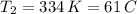Chemistry, 27.08.2019 03:30 lindseyr190
Chloroform, chcl3, was once used as an anesthetic. in spy movies it is the liquid put in handkerchiefs to render victims unconscious. its vapor pressure is 197 mmhg at 23 degrees c and 448 mmhg at 45 degrees
c. estimate its
a. heat of vaporization
b. normal boiling point i calculated the heat of vaporization to be 29.3 kj/mol. i'm having some trouble figuring out the normal boiling point, however. i know that the normal boiling point is when, at 1 atm, a liquid boils at a temperature at which its vapor pressure is equal to the pressure above its surface. so p1=p2=1 atm, if p1= vapor pressure and p2= atmospheric pressure/pressure above surface. i figured i could plug this into pv=nrt and solve, but i'm not given a lot of information. i considered assigning arbitrary values for n and v, so i would have
t= (1.00 atm)(1.00 l)/(1.00 ) but is that really the best way to do this problem, or would it even work at all?
i would think you could use the clausius-clapeyron equation, just as you did for delta h vap, but this time one of the ps will be 760 mm and calculate t for that p.
you, that makes sense, but how do i account for p2 and t2 in the equation if i don't know those values either?
but you have two vapor pressures at two temperatures. i would pick 23 c (change to kelvin, of course) and 197 mm for t1 and p1. then 760 mm and t2 for the others. you have all of the other numbers. check my thinking.
oh, of course, i had completely forgotten about that. you.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, bryneosburn
Lem 2 the data below are for the system ethyl propyl ether (1)-chloroform (2) at 0.5 bar. use the data to answer the following questions (all questions refer to p d 0: 5 bar). a) what are the boiling points of the pure components at 0.5 bar? b) a mixture with the overall composition z1 d 0: 1 is brought to 47.6ä±c, 0.5 bar. what is the phase? c) 100 mole of a mixture with z1 d 0: 1 (state a) is mixed with 22 mole of pure ethyl propyl ether vapor (state b). the mixing takes place at 47.6 ä±c, 0.5. bar. what is the phase of the resulting mixture (state c)? if the state is a v/l mixture report the number of moles and mole fractions in each phase. d) plot the txy graph and show states a, b and c. the graph must be done by computer and should be properly annotated. ethyl propyl ether (1) - chloroform (2) at 0.5 bar t ( ä±c) x1 y1 t ( ä±c) x1 y1 42.9 0.000 0.000 49.0 0.470 0.455 43.0 0.020 0.010 49.1 0.520 0.520 43.9 0.065 0.029 48.9 0.567 0.592 45.4 0.156 0.089 48.3 0.652 0.720 46.4 0.215 0.142 47.6 0.745 0.815 47.6 0.296 0.223 46.7 0.822 0.872 48.3 0.362 0.302 45.7 0.907 0.937 48.7 0.410 0.375 44.6 1.000
Answers: 3

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 17:50, kaylamount
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2
You know the right answer?
Chloroform, chcl3, was once used as an anesthetic. in spy movies it is the liquid put in handkerchie...
Questions in other subjects:

English, 21.10.2020 16:01

Computers and Technology, 21.10.2020 16:01






Mathematics, 21.10.2020 16:01


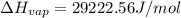
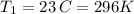
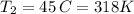
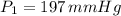
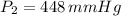
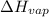 : heat of vaporization
: heat of vaporization