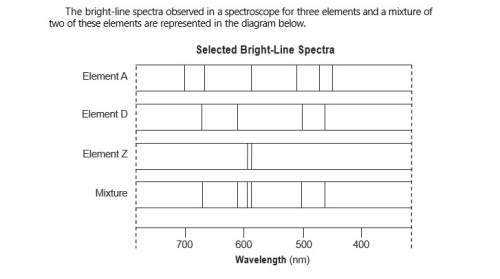
Describe, in terms of both electrons and energy states, how the light represented by
the spect...

Chemistry, 26.12.2019 06:31 perezshayla56
Describe, in terms of both electrons and energy states, how the light represented by
the spectral lines is produced.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:00, angeljohnson2081
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 18:30, ashleymer384
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 11.11.2020 20:00

Business, 11.11.2020 20:00

English, 11.11.2020 20:00

Mathematics, 11.11.2020 20:00

Biology, 11.11.2020 20:00


Mathematics, 11.11.2020 20:00





