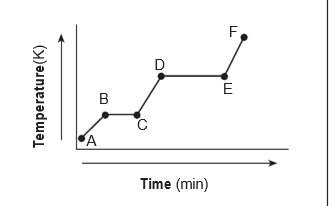
The heating curve below represents a sample of
a substance starting as a solid below its melti...

The heating curve below represents a sample of
a substance starting as a solid below its melting
point and being heated over a period of time.
which statement describes the energy of the
particles in this sample during interval de?
(1) both potential energy and average kinetic
energy increase.
(2) both potential energy and average kinetic
energy decrease.
(3) potential energy increases and average
kinetic energy remains the same.
(4) potential energy remains the same and
average kinetic energy increases.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

You know the right answer?
Questions in other subjects:


Biology, 29.03.2021 18:30










