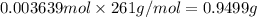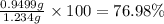The concentration of barium ions in any solution can also be determined via gravimetric analysis. an impure sample of barium nitrate with a mass of 1.234 g, is completely dissolved in water and the resulting solution is reacted with an excess of aqueous sodium sulfate. a precipitate forms, and after filtering and drying, it was found to have a mass of 0.848 g.
a) what is the relevance of adding eccess sodium sulfate?
b) calculate the % of barium nitrate in the original 1.234 g sample.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
You know the right answer?
The concentration of barium ions in any solution can also be determined via gravimetric analysis. an...
Questions in other subjects:

Mathematics, 19.08.2019 06:20

Mathematics, 19.08.2019 06:20

English, 19.08.2019 06:20

History, 19.08.2019 06:20

Mathematics, 19.08.2019 06:20

Mathematics, 19.08.2019 06:20

Mathematics, 19.08.2019 06:20

English, 19.08.2019 06:20

History, 19.08.2019 06:20

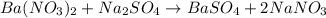
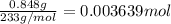
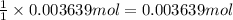 of barium nitrate.
of barium nitrate.