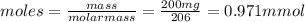Asap
1. ibuprofen (c13h18o2) is the active ingredient in many nonprescription pain relievers....

Chemistry, 04.02.2020 12:58 savannahvargas512
Asap
1. ibuprofen (c13h18o2) is the active ingredient in many nonprescription pain relievers. each tablet contains 200 mg of ibuprofen, and a typical adult dose is two tablets every six hours.
• determine the molar mass of ibuprofen. show all steps to find the answer.
• calculate the number of moles of ibuprofen in a single tablet. show all steps to find the answer.
• calculate the number of moles of ibuprofen that an adult would have taken if she took four doses of ibuprofen in one day. show all steps to find the answer.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:02, Killion2022
25 m/s to miles/hr can anyone solve it? it’s for chemistry
Answers: 2


You know the right answer?
Questions in other subjects:


Mathematics, 24.01.2021 04:20

English, 24.01.2021 04:20

Mathematics, 24.01.2021 04:20

Mathematics, 24.01.2021 04:20

Mathematics, 24.01.2021 04:20


Social Studies, 24.01.2021 04:20


English, 24.01.2021 04:20