Charles law
a balloon of helium was put in the freezer at -23°c. its volume at this temp...

Chemistry, 28.01.2020 01:31 jessysmith9678
Charles law
a balloon of helium was put in the freezer at -23°c. its volume at this temperature was 2.5 liters. it was removed from the freezer. eventually, it reached a temperature of 177°c. what would its volume be at this temperature? (neglect any force used to stretch the rubber balloon.) asap
a. 1.4
b. 45000
c. 4.5
d. 0.22

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, irene4523
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 21.06.2019 21:00, Gghbhgy4809
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1
You know the right answer?
Questions in other subjects:


Computers and Technology, 28.07.2020 22:01



Mathematics, 28.07.2020 22:01





Biology, 28.07.2020 22:01

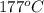 will be, (C) 4.5 L
will be, (C) 4.5 L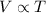

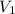 = initial volume of gas = 2.5 L
= initial volume of gas = 2.5 L = final volume of gas = ?
= final volume of gas = ? = initial temperature of gas =
= initial temperature of gas = 
 = final temperature of gas =
= final temperature of gas = 




