1. avogadro's number tells us the number of particles (atoms, molecules, ions) in one
2...

Chemistry, 05.10.2019 07:40 hellokitty1647
1. avogadro's number tells us the number of particles (atoms, molecules, ions) in one
2. what is the molar mass of caffeine (c₈h₁₀n₄o₂)?
3. what coefficient would go in front of alcl₃ to balance this equation?
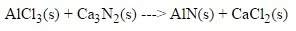

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:00, williedenmark42
What is the most common form of matter in the universe
Answers: 2

Chemistry, 23.06.2019 04:31, mdarter
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
Questions in other subjects:


Mathematics, 27.04.2021 15:00




Mathematics, 27.04.2021 15:00






