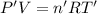Arigid, 2.50 l bottle contains 0.458 mol he. the pressure of the gas inside the bottle is 1.83 atm. if 0.713 mol ar is added to the bottle and the pressure increases to 2.05 atm, what is the change in temperature of the gas mixture? the initial temperature of the gas is k.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1


Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
Arigid, 2.50 l bottle contains 0.458 mol he. the pressure of the gas inside the bottle is 1.83 atm....
Questions in other subjects:


Mathematics, 05.02.2021 03:00



Social Studies, 05.02.2021 03:00

Mathematics, 05.02.2021 03:00

Mathematics, 05.02.2021 03:00

Spanish, 05.02.2021 03:00


 ( Ideal gas equation)
( Ideal gas equation)