
Chemistry, 07.10.2019 08:02 ilovejustinbieber42
Calculate the molar solubility of copper(ii) arsenate (cu3(aso4)2) in water. use 7.6 x 10^-36 as the solubility product constant of cu3(aso4)2.
9.1 x 10^-4 m
3.4 x 10^-2 m
3.7 x 10^-8 m
8.7 x 10^-2 m

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
Calculate the molar solubility of copper(ii) arsenate (cu3(aso4)2) in water. use 7.6 x 10^-36 as the...
Questions in other subjects:


Mathematics, 18.02.2021 02:30

Mathematics, 18.02.2021 02:30

Biology, 18.02.2021 02:30





Mathematics, 18.02.2021 02:30

Mathematics, 18.02.2021 02:30

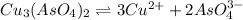
 will be given by:
will be given by:
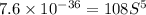
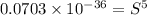
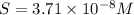
 .
.

