Answers: 1


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 10:00, LlayahHarbin
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 2
You know the right answer?
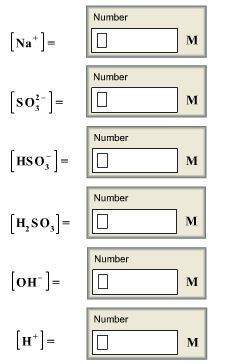
Calculate the concentrations of all species in a 1.70 m na2so3 (sodium sulfite) solution. the ioniza...
Questions in other subjects:





Geography, 20.12.2019 23:31